Elimination of Guesswork with the BioFire® FilmArray® System (bioMérieux)
The BioFire System performs syndromic PCR tests that detect infectious agents at the molecular level. With a revolutionary syndromic approach and the broadest target menu on the market, the bioMérieux or BioFire® FilmArray® Panels empower healthcare providers to choose the right test, the first time.
Traditional infectious diseases diagnostic procedures leave many infections undiagnosed, which result in downstreaming of tests and procedures, compromised care and patient dissatisfaction.
What is the new name for Biofire?
The new name for Biofire is bioMérieux. For several years BioFire was continuing to maintain its brand identity and operations. BioFire was acquired in 2014 by bioMérieux SA, which they claim being a global leader in in vitro diagnostics for more than 55 years.
They
then reached a critical point in our development where it made sense to them to
align more closely with bioMérieux,” explains Andrea Kendell, CFO North America
& VP Finance Global Manufacturing for bioMérieux. “What this means, is that
beginning in January 2022, BioFire products will be folded into the bioMérieux
product portfolio. As a company, it will simply be known as bioMérieux.”
What are BioFire® FilmArray® Panels?
Biofire or bioMérieux panels are which are Comprehensive Panels giving Better Diagnostics
The
BioFire Panels test for bacteria, parasites, viruses, yeast and antimicrobial
resistance genes. Whether one desires to select appropriate therapy for septic
patients or determine exactly which respiratory pathogen is making a young
child ill, the BioFire® FilmArray® Systems can produce answers really
quick.
Designed
with the syndromic approach, each panel can detect a broad grouping of
probable pathogenic causes in a single and rapid test.
Comprehensive
panels remove the scope of guess work, giving laboratories and healthcare
providers a higher probability value of identifying pathogens associated with
infectious diseases. Product availability varies form country to country. You
need to consult your bioMérieux representative in order to procure it.
What is The BioFire® Respiratory 2.1?
(RP2.1)
Panel
The
FDA De Novo authorized BioFire RP2.1 Panel is known as a frontline test to help
clinicians quickly diagnose respiratory infections taking place, including
COVID-19, influenza, RSV and many more.
SARS-CoV-2
is one of the top concerns for patients and clinicians, but several respiratory
pathogens can cause nearly non-distinguishable symptoms.
1 Test. 22 Targets. -45 Minutes
The
BioFire RP2.1 Panel uses a syndromic type approach to accurately detect and
identify the pathogens in most common association with respiratory infections.
Rapid respiratory PCR test results most probably enable better-informed diagnosis and treatment of patients.
Fast and comprehensive BioFire RP2.1 Panel offers a run time of about 45 minutes, thereby enabling high efficiency and throughput on the BioFire® FilmArray® 2.0 and the BioFire® FilmArray® Torch Systems.
Quick turnaround on a broad menu of pathogens may also help the clinicians
make vital decisions regarding admission, isolation, cohorting and additional
diagnostic testing.
THE BIOFIRE RESPIRATORY 2.1 PANEL MENU
Overall
97.1% sensitivity & 99.3% specificity (prospective specimens) 1
SARS-CoV-2
98.4% PPA and 98.9% NPA2
Sample
Type: Nasopharyngeal swab in transport media or saline
Which are Diagnosable Viruses by BioFire® FilmArray® System?
●
Adenovirus
●
Coronavirus 229E
●
Coronavirus HKU1
●
Coronavirus NL63
●
Coronavirus OC43
●
Human Metapneumovirus
●
Human Rhinovirus/Enterovirus
●
Influenza A virus
●
Influenza A virus A/H1
●
Influenza A virus A/H3
●
Influenza A virus A/H1-2009
●
Influenza B virus
●
Parainfluenza virus 1
●
Parainfluenza virus 2
●
Parainfluenza virus 3
●
Parainfluenza virus 4
●
Respiratory syncytial virus
●
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
BACTERIA:
●
Bordetella Parapertussis
●
Bordetella Pertussis
●
Chlamydia Pneumoniae
●
Mycoplasma Pneumoniae
Value of the Syndromic Approach and COVID-19
In
few cases, as many as 20% of COVID-19 patients have co-infections with some
other respiratory viruses.
As
respiratory symptoms are quiet similar and also overlap, a syndromic panel can
provide fast, comprehensive answers and wedd the guesswork out of choosing for
which pathogens to test.
Study
results usually suggest higher rates of co-infections between SARS-CoV-2 and
other respiratory pathogens than previously reported.
How to Improve Clinical Outcomes through Biofire?
The
BioFire® FilmArray® Respiratory Panels have been proving to significantly
reduce ICU days3 and duration of antibiotic use. Also, to optimize patient
management with clinically actionable results.
Biofire Solutions For Labs
Faster
Results. Improved Workflow.
The
BioFire® FilmArray® System is scalable and easy to use. This enables its easy
integration into clinical laboratories.
This
helps to streamline lab workflow and maximize productivity with multiplex PCR
testings with infectious disease diagnostics from the BioFire System solution.
What is Syndromic Testing with BioFire System?
The
BioFire System combines speed, ease of use and a growing portfolio of
comprehensive panels into one sample answer system.
BioFire
offers a syndromic infectious disease testing approach that targets a broad
menu of possible pathogens in single rapid test. Because of the ease of its
use, BioFire System can be used around the clock, eliminating the necessity to
delay or send out certain tests.
Also,
BioFire’s molecular diagnostics may enable long time cost savings as comparable
with traditional testing solutions available.
Fast
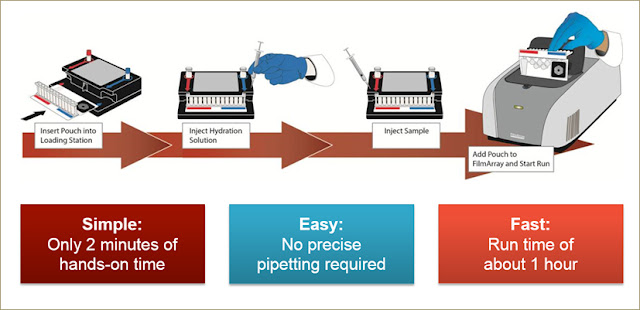
The
BioFire® FilmArray® Panels have a run-time of about 1hr, allowing labs to
provide answers to physicians in a time frame of clinical relevance.
Easy
The
BioFire System takes only 2 minutes of hands-on lab technician time. This
is without precise measuring or pipetting.
The
sample is automatically analyzed and results are reported in an easily readable
form.
Comprehensive
The
comprehensive BioFire Panels are also helpful in detecting co-infections as
well as uncommon pathogens.
Syndromic
molecular diagnostics are able to help labs in streamlining their workflow by
reducing serial or send-out testing.
How The BioFire FilmArray System Works?
Each BioFire Panel pouch stores all the necessary reagents for sample preparation, reverse transcription-PCR, PCR and detection in a freeze-dried format.
During a run, the BioFire System extracts and purifies all nucleic acids from the unprocessed sample. Then it performs a nested multiplex PCR.
During the first stage, the system performs a single, large-volume, multiplexed reaction.
Finally, individual single-plex, second-stage PCR reactions detect the products
from the first-stage PCR.
Using endpoint melting curve data, the BioFire System software automatically analyzes the result for each target.
When the run is complete, the software reports
whether or not each pathogen is detected in the sample.
BioFire
System test run steps:
1. SETUP
Inject hydration solution and an unprocessed sample.
2. TEST
Insert the pouch into the BioFire System unit and start the run.
3. GET
RESULT The software reports whether or not each pathogen is detected in the
sample.
What is BioFire Syndromic Trends?
BioFire Syndromic Trends (Trend) is a software feature from BioFire that provides local and regional pathogen circulation trends on demand.
Users can view pathogen
trends for their lab as well as regional and national pathogen trends, all of
which are created from de-identified data that has been aggregated with other
participating institutions’ test data.
BioFire
Trend delivers automatic weekly email reports and allows users to explore their
data using BioFire Trend’s online dashboard, which enables users to further
explore their data by location, laboratory, or even chart type.
BioFire Syndromic Trends Features
• Downloadable
custom reports
• De-identified
data to protect patient privacy
• Weekly
notifications for local and regional activity
• Near
real-time data collection and historical data access
• Unlimited
licenses to clinical website for data collection
How Do I Join the BioFire Syndromic Trends Network?
This
feature is available to BioFire customers, and connecting takes just minutes.
Get the most out of your syndromic testing by signing up to join the BioFire
Syndromic Trends Network today.
What are Biofire Solutions for Customers?
1st
in Diagnostics, 1st in Customer Care
BioFire
understands that ongoing management of a diagnostics platform in the clinical
laboratory or in clinic-based settings is not a trivial task. With that in
mind, Biofire aims to provide easy access to information and resources to help
current customers use BioFire products and services with complete confidence.
Does Biofire have World Class Support Team?
The
BioFire Support team is comprised of several highly trained, experienced groups
dedicated to client success.
Customer Technical Support of Biofire
With
the urgent nature of patient and community care, Biofire takes pride in
addressing all concerns quickly and accurately.
BioFire
is dedicated in provision of world class customer support 24 hours a
day, 7 days a week, 365 days in a year.
For
assistance you may contact Biofire customer technical support team
at:
• support@biofiredx.com
• 801-736-6354
option 5
• 1-800-735-6544
Who are Biofire Field Application Specialists?
Biofire
Field Application Specialists are specialized BioFire employees who ensure
customer satisfaction during the initial installation and verification process
of BioFire® FilmArray® System.
Biofire
Field Application Specialist will provide appropriate training and ensure your
team is ready to use BioFire System and panels.
Who are Biofire Medical Education Liaisons?
Biofire
Medical Education Liaisons are those who work directly with your clinicians and
hospital staff in optimizing the use of the BioFire System in client’s
facility. This may include working with Antimicrobial Stewardship Program and
Infection Control Committee as clients change their protocols and clinical
pathways in response to earlier pathogen identification and clinical diagnosis
by the use of our BioFire® FilmArray® Panels.
Biofire Frequently Asked Customer Questions - FAQs
Q.
How do I place an order or check on my existing order?
Ans.
You may contact Customer Service at salesorders@biofiredx.com or by phone at:
•
+1-801-736-6354 x1502
•
International Sales No.: +1-801-736-6354 x1502
Q. Where can I find Safety Data Sheets?
Ans. All
of our SDS can be found on the Documents Page under Support.
User
Guide
Product
specific manuals are listed in the Instructions for Use and Manuals section of
the documentation page and will include links to the electronic labeling site
for your convenience.
System
maintenance is complete, latest documentation version, customers can choose to
download in multiple languages.
CLSI Program Format Template
BioFire
understands that technical procedures must be clear. We provide BioFire Panel
program templates in CLSI format. Customers can download these templates and
customize them for the specific needs of their labs.
Technical Description
Technical
notes are a form of communication that BioFire uses to provide information to
customers. These documents, generated by the customer's technical support team,
can be an invaluable resource in the laboratory.
Technical
notes can be downloaded from the documentation section of the website.
What is Biofire's mission and values?
BioFire
simplifies the lives of our customers with innovative, easy-to-use clinical
molecular diagnostic solutions that deliver reliable results.
What are BioFire's combination therapies that are transforming infectious disease diagnostics?
Many infections have similar signs and symptoms. However, today's targeted infectious disease diagnostics limit testing to the most common pathogens associated with clinical syndromes. As a result, most infections go undiagnosed, leading to additional downstream testing, patient dissatisfaction and disruption to patient care. BioFire's approach to infectious disease diagnostics changes all that.
The
syndromic approach is a symptom-based diagnostic approach that combines a wide
range of possible etiologies into one rapid test. This makes it easy for
doctors to choose the right test the first time.
Biofire’s innovative
teams are leading the industry in infectious disease diagnostics, offering
molecular solutions to reduce time to medical results, enable healthcare
professionals to make better diagnostic decisions and reduce healthcare costs.
Biofire
are committed to improving the quality of life for everyone, everywhere and
every day.
Author's Bio
Education: MBBS, MD
Occupation: Medical Doctor
Specialization: Community Medicine, General Surgery, Natural Treatment
Experience: 18 Years as a Medical Practitioner
Share this in Public Information Interest>>




Comments
Post a Comment